The Morgan Adams Foundation is proud to partner with the Osteosarcoma Institute (OSI) to fund a correlative study alongside a new Phase 2 clinical trial for kids with advanced high-grade osteosarcoma.
OSI and The Morgan Adams Foundation

The Osteosarcoma Institute (OSI) was founded in 2017. Its mission is to dramatically increase treatment options and survival rates in osteosarcoma patients by identifying and funding the most promising and breakthrough osteosarcoma clinical trials and science.
The Morgan Adams Foundation has been expanding its reach and impact across all areas of pediatric cancer research since its inception in 2001. We have known many kids and young adults who have been diagnosed with osteosarcoma. Only half of them are still alive, and we decided to join forces with OSI in 2025 to help in the effort to improve the outlook for kids diagnosed with osteosarcoma.
About Osteosarcoma
Osteosarcoma is the most common bone cancer diagnosed in children, teens, and young adults between 10 and 30 years old. There is a peak incidence of osteosarcoma diagnosis during the adolescent growth spurt. It can occur anywhere along the skeleton, but the most common sites are in longer bones, including the knee and shoulder.
There are approximately 1,000 new cases of osteosarcoma diagnosed in the United States each year, and more than 26,000 globally. Current therapy for osteosarcoma includes a combination of standard chemotherapy and surgery. Most patients receive chemotherapy for several months before surgery and resume chemotherapy after they have recovered from surgery.
The type of surgery varies, but commonly includes removal of the primary tumor and, if possible, limb-preservation surgery. Surgery often involves the use of an internal prosthesis or, rarely, an external prosthesis. Physical therapy is an important step following recovery from surgery to ensure the patient’s strength and movement are regained safely.
The outlook for osteosarcoma patients depends on many factors, including the location and size of the tumor, whether the cancer has spread, and the person’s age and overall health. Devastatingly, about a third of all cases will relapse and spread to other areas of the body, such as the lungs. When osteosarcoma cells spread to other parts of the body, survival rates drastically reduce from ~76% to ~24%.
There have been minimal improvements in the survival of osteosarcoma patients for more than 40 years. Osteosarcoma is complex, and more research is urgently needed to improve outcomes and reduce the late effects caused by toxic chemotherapies.
Phase 2 Clinical Trial
OSI is funding a new Phase 2 clinical trial investigating OMO-103, a Myc-targeting drug developed by Peptomyc. Myc is an oncogene that is commonly amplified in osteosarcoma and is associated with poor outcomes for patients. Because Myc plays a key role in tumor development and progression, it is a critical target for therapy. OMO-103 has proven to be effective in targeting Myc and killing cancer cells in preclinical studies and Phase 1 trials for solid tumors.
Led by Dr. Claudia Valverde Morales at Vall d’Hebron Institute of Oncology in Spain, the Phase 2 trial will enroll 10 patients with advanced high-grade osteosarcoma who have received at least one prior treatment. The primary objective is to assess progression-free survival at 16 weeks, providing key data on the drug’s safety and anti-tumor activity.
The Morgan Adams Foundation’s Role
To complement the clinical trial, The Morgan Adams Foundation is funding a correlative study led by Brian Crompton, MD and his team at the Dana-Farber Cancer Institute in Boston, Massachusetts.
An important and desired outcome of the OMO-103 clinical trial is hypothesis-generating correlative analyses utilizing liquid biopsies, non-invasive medical tests that analyze bodily fluids for signs of disease.
Dr. Crompton’s laboratory will receive, process, and analyze blood samples from clinical trial participants to understand how the drug being tested, OMO-103, affects the tumor and levels of Myc. Samples will be collected at 6 timepoints throughout the trial treatment and will assess the cell-free tumor DNA burden and Myc signature, as well as the circulating tumor cell pharmacodynamics.
According to Dr. Crompton, “Liquid biopsy technologies have been employed for numerous purposes that have improved the care of patients with solid tumors, including early detection, pre-treatment prognostication, precision medicine, measures of treatment response, detection of minimal residual disease and relapse, and the study of tumor evolution. In pediatrics, numerous studies (primarily from the Crompton lab) now show that circulating tumor DNA (ctDNA) levels and detection of specific variants at diagnosis are prognostic. In addition, changes in ctDNA levels early on-treatment also appear to be associated with outcome.”
This trial and Dr. Crompton’s correlative study will help make progress toward the ultimate goal of introducing a new and effective treatment for osteosarcoma, offering hope for children and young adults facing this devastating disease.
What is a correlative study?
A correlative study aims to identify biological characteristics that are linked to a patient’s response—or lack of response—to a particular therapy in a clinical trial.
By examining these correlations, researchers hope to develop biomarkers (measurable indicators of underlying biology) that can more precisely predict which patients are likely to benefit from the treatment and which are not.
While clinical trials typically aim to determine whether an experimental therapy is effective for the entire group that enters a trial, they often lack funding to investigate why the treatment works or does not work and identify subsets of the trial’s participants that respond differently.
Correlative studies can provide valuable insights, enhancing the understanding needed to guide future developments in treatment.
This exciting collaboration with the Osteosarcoma Institute is only possible because of the support of generous donors. Thank you for helping give kids with cancer a brighter future!
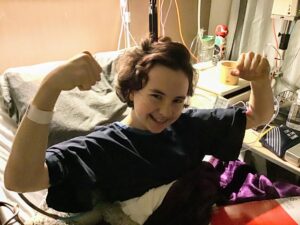
Meet Ally
Ally was just about to start her freshman year of high school when she noticed a growing bump on her lower left leg. After a biopsy and countless scans, Ally was diagnosed with osteosarcoma.
After nearly a year of treatment, including 9 months of chemotherapy and a handful of surgeries, Ally was officially in remission. Since then, Ally has undergone two more surgeries to reconstruct her left leg.
Today, a little over 3 years out of her last surgery, with 3-inch, 10-inch and 13-inch scars decorating her leg, Ally lives a happy, healthy life. Her leg still affects her daily, but she is forever grateful she still has a leg, is able to function, and enjoy life to its fullest.
If you would like to make a gift to support groundbreaking kids’ cancer research in 2025, click here.
Your donation is the backbone of the search for new treatments for kids with cancer and supports all of the ways The Morgan Adams Foundation is working to end pediatric cancer.